Will Taking Testosterone Reduce Gynecomastia
ABSTRACT
Gynecomastia is a relatively common disorder. Its causes range from benign physiological processes to rare neoplasms. In order to properly diagnose the etiology of the gynecomastia, the clinician must understand the hormonal factors involved in breast development. Parallel to female breast development, estrogen, GH, and IGF-1 are required for breast growth in males. Since a balance exists between estrogen and androgens in males, any disease state or medication that can increase circulating estrogen or decrease circulating androgen, causing an elevation in the estrogen to androgen ratio, can induce gynecomastia. Due to the diversity of possible etiologies, including a neoplasm, performing a careful history and physical is imperative. Once gynecomastia has been diagnosed, treatment of the underlying cause is warranted. If no underlying cause is discovered, then close observation is appropriate. If the gynecomastia is severe and of recent onset, however, medical therapy can be attempted and if ineffective, glandular tissue can be removed surgically. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
This chapter reviews the ontogeny and physiology of breast development; factors that influence breast enlargement in the male; the differential diagnosis of gynecomastia; the process of diagnostic investigation; and treatment of gynecomastia.
BREAST ONTOGENY AND DEVELOPMENT
Male breast development occurs in an analogous fashion to female breast development. At puberty in the female, complex hormonal interplay occurs resulting in growth and maturation of the adult female breast.
In early fetal life, epithelial cells, derived from the epidermis of the area programmed to become the areola, proliferate into ducts, which connect to the nipple at the skin's surface. The blind ends of these ducts bud to form alveolar structures in later gestation. With the decline in fetal prolactin and placental estrogen and progesterone at birth, the infantile breast regresses until puberty (1).
During thelarche in females, the initial clinical appearance of the breast bud and growth and division of the ducts occur, giving rise to club-shaped terminal end buds, which then form alveolar buds. Approximately a dozen alveolar buds will cluster around a terminal duct, forming the type 1 lobule. The type 1 lobule will mature into types 2 and 3 lobules, called ductules. The number of alveolar buds increases to as many as 50 in type 2 and 80 in type 3 lobules. The entire differentiation process takes years after the onset of puberty and, if pregnancy is not achieved, may never be completed (2).
HORMONAL REGULATION OF BREAST DEVELOPMENT
The initiation and progression of breast development involves a coordinated effort of pituitary and ovarian hormones, as well as local mediators (Figure 1).

Figure 1.
Hormones Affecting Growth and Differentiation of Breast Tissue.
Estrogen, GH and IGF-1, Progesterone, and Prolactin
Estrogen and progesterone act in an integrative fashion to stimulate normal adult female breast development. Estrogen, acting through its ER receptor, promotes ductal growth, while progesterone, acting through its receptor (PR), promotes alveolar development (1). This is demonstrated by experiments in ER knockout mice that display grossly impaired ductal development, whereas PR knockout mice possess significant ductal development, but lack alveolar differentiation (3, 4).
Although estrogens and progestogens are vital to mammary growth, they are ineffective in the absence of anterior pituitary hormones (5). Thus, neither estrogen alone nor estrogen plus progesterone can sustain breast development without other mediators, such as GH and IGF-1. This was confirmed by studies involving the administration of estrogen and GH to hypophysectomized and oophorectomized female rats, which resulted in breast ductal development. The GH effects on ductal growth are mediated through stimulation of IGF-1. This is demonstrated by studies of estrogen and GH administration to IGF-1 knockout rats that showed significantly decreased mammary development when compared to age-matched IGF-1- intact controls. Combined estrogen and IGF-1 treatment in these IGF-1 knockout rats restored mammary growth (6, 7). In addition, Walden et al. demonstrated that GH-stimulated production of IGF-1 mRNA in the mammary gland itself, suggesting that IGF-1 production in the stromal compartment of the mammary gland acts locally to promote breast development (8). Furthermore, other data indicates that estrogen promotes GH secretion and increases GH levels, stimulating the production of IGF-1, which synergizes with estrogen to induce ductal development. In a population-based study of healthy school boys and adolescents, IGF-I levels were found to be elevated in boys with pubertal gynecomastia compared with boys without gynecomastia, suggesting that the GH-IGF-I axis may be involved in the pathogenesis of pubertal gynecomastia (9).
Progesterone has minimal effects in breast development without concomitant anterior pituitary hormones, indicating that progesterone also interacts closely with pituitary hormones. For example, prolonged treatment of dogs with progestogens, such as depot medroxyprogesterone acetate or with proligestone, caused increased GH and IGF-1 levels, suggesting that progesterone may also have an effect on GH secretion (10). In addition, clinical studies have correlated maximal cell proliferation to specific phases in the female menstrual cycle. For example, maximal proliferation occurs not during the follicular phase when estrogens reach peak levels and progesterone is low (less than 1 ng/mL [3.1nmol]), but rather, it occurs during the luteal phase when progesterone reaches levels of 10-20 ng/mL (31- 62nmol) and estrogen levels are two to three times lower than in the follicular phase (11). Furthermore, immunohistochemical studies of ER and PR showed that the highest percentage of proliferating cells, found almost exclusively in the type 1 lobules, contained the highest percentage of ER and PR positive cells (2). Similarly, there is immunocytological presence of ER, PR, and androgen receptors (AR) in gynecomastia and male breast carcinoma. ER, PR and AR expression was observed in 100% (30/30) of gynecomastia cases (12). Given these data and the fact that PR knockout mice lack alveolar development in breast tissue, it appears that progesterone, analogous to estrogen, increases GH secretion and acts through its receptor on mammary tissue to enhance breast development, specifically alveolar differentiation (13, 4).
Prolactin is another anterior pituitary hormone integral to breast development. Prolactin is not only secreted by the pituitary gland but may be produced in normal mammary tissue epithelial cells and breast tumors (14, 15). Prolactin stimulates epithelial cell proliferation only in the presence of estrogen and enhances lobulo-alveolar differentiation only with concomitant progesterone. Galactorrhea is seen rarely in hyperprolactinemia, possibly because of the low estrogen levels as a result of suppression of LH secretion. Previously, receptors for luteinizing hormone/ human chorionic gonadotropin have been found in both male and female breast tissues, but their functional roles remain to be determined (16).
Androgen and Aromatase
Estrogen effects on the breast might be the result of circulating estradiol levels or locally produced estrogens. Aromatase P450 catalyzes the conversion of the C19 steroids, androstenedione, testosterone, and 16−α−hydroxyandrostenedione to estrone, estradiol-17β, and estriol. As such, an overabundance of substrate or an increase in enzyme activity can increase estrogen concentrations and initiate the cascade to breast development in females and males. For example, in the more complete forms of androgen insensitivity syndromes in genetically male (XY) patients, excess androgen aromatizes into estrogen resulting in not only gynecomastia, but also a phenotypic female appearance. Furthermore, the biologic effects of over expression of the aromatase enzyme in female and male mice transgenic for the aromatase gene result in increased breast proliferation. In female transgenics, over expression of aromatase promotes the induction of hyperplastic and dysplastic changes in breast tissue. Over expression of aromatase in male transgenics causes increased mammary growth and histological changes similar to gynecomastia, an increase in estrogen and progesterone receptors, and an increase in downstream growth factors such as TGF-beta and βFGF (17). Interestingly, treatment with an aromatase inhibitor leads to involution of the mammalian gland phenotype (18). Thus, although androgens do not stimulate breast development directly, they may do so if they aromatize to estrogen. This occurs in cases of androgen excess or in patients with increased aromatase activity.
PHYSIOLOGIC GYNECOMASTIA
Gynecomastia, breast development in males, can occur normally during three phases of life. The first occurs shortly after birth in both males and females. This is partly caused by the high levels of estradiol and progesterone produced by the mother during pregnancy, which stimulates breast tissue in the newborn. Another mechanism is in part due to the increased conversion of steroid hormone precursors to sex steroids and a neonatal surge of gonadotropins. It can persist for several weeks after birth and can cause mild breast discharge called "witch's milk" (2). Puberty marks the second situation in which gynecomastia can occur physiologically. In fact, up to 60% of boys have clinically detectable gynecomastia by age 14. Although it is mostly bilateral, it is often asymmetrical and can occur unilaterally. Pubertal gynecomastia usually resolves within 3 years of onset (2).
In early puberty, the pituitary gland releases gonadotropins at night and stimulates testicular production of testosterone during the very early morning hours. Estrogens, however, rise throughout the entire day. Some studies have shown that a decreased androgen to estrogen ratio exists in boys with pubertal gynecomastia when compared with boys who do not develop gynecomastia (19). Furthermore, another study showed increased aromatase activity in the skin fibroblasts of boys with gynecomastia. Thus, the mechanism by which pubertal gynecomastia occurs may be due to either decreased production of androgens or increased aromatization of circulating androgens, thus increasing the estrogen to androgen ratio (20).
The third age range in which gynecomastia is frequently seen is during older age (>60 years). Although the exact mechanisms by which this can occur have not been fully elucidated, evidence suggests that it may result from increased peripheral aromatase activity secondary to the increase in total body fat, relatively elevated LH concentrations, and a decrease in serum testosterone concentrations associated with male aging. For instance, investigators have shown increased urinary estrogen levels in obese individuals, and have demonstrated aromatase expression in adipose tissue (21). Thus, like the gynecomastia of obesity, the gynecomastia of aging may partly result from increased aromatase activity, causing increased conversion of androgens to estrogens (22). Moreover, not only does total body fat increase with age, but there may be an increase in aromatase activity in the adipose tissue already present, increasing circulating estrogens even further. SHBG increases with age in men. Since SHBG binds estrogen with less affinity than testosterone, the bioavailable estradiol to bioavailable testosterone ratio may increase in the obese older male. Lastly, elderly patients may take multiple medications associated with gynecomastia. One cohort study suggests medications are at least partially responsible in 80% of cases (23).
PATHOLOGIC GYNECOMASTIA
Pathologic gynecomastia is due to an increase in the circulating and/or local breast tissue ratio of estrogen to androgen.
Increased Estrogen
Since the development of breast tissue in males occurs in an analogous manner to that in females, the same hormones that affect female breast tissue can cause gynecomastia. In post-pubertal boys and adult men, the testes secrete 6-10 µg of estradiol and 2.5 µg of estrone per day. Since this only comprises a small fraction of estrogens in the circulation (i.e. 15% of estradiol and 5% of estrone), the remainder of estrogen in men is derived from the extraglandular aromatization of testosterone and androstenedione to estradiol and estrone (24). Thus, any cause of estrogen excess from overproduction or peripheral aromatization of androgens can initiate the cascade to breast development.
TUMORS
Testicular tumors can lead to increased blood estrogen levels by estrogen overproduction, androgen overproduction with aromatization in the periphery to estrogens, and by ectopic secretion of gonadotropins which stimulate otherwise normal Leydig cells. Tumors causing an overproduction of estrogen represent an unusual but important cause of estrogen excess. Examples of estrogen-secreting tumors include: Leydig cell tumors, Sertoli cell tumors, granulosa cell tumors, and adrenal tumors.
Interstitial cell tumors, or Leydig cell tumors constitute 1%-3% of all testis tumors. Usually, they occur in men between the ages of 20 and 60, although up to 25% of them occur prepubertally. In prepubertal cases, isosexual precocity, rapid somatic growth, and increased bone age with elevated serum testosterone and urinary 17-ketosteroid levels are the presenting features. In adults, elevated estrogen levels coupled with a palpable testicular mass and gynecomastia suggests a testicular tumor. Of note, some testis tumors may only be apparent in ultrasound because of the small size; some may produce testosterone and do not cause gynecomastia. Though mostly benign, Leydig cell tumors may be malignant and metastasize to lung, liver, and retroperitoneal lymph nodes (25, 26).
Sertoli cell tumors comprise less than 1% of all testicular tumors and occur at all ages, but one third have occurred in patients less than 13 years, usually in boys under 6 months of age. Although they arise in young boys, they usually do not produce endocrine effects in children. Again, the majority are benign, but up to 10% are malignant. Gynecomastia occurs in one third of cases of Sertoli cell tumors, presumably due to increased estrogen production (26).
Granulosa cell tumors, occurring very rarely in the testes, can also overproduce estrogen. Gynecomastia at presentation was reported in some cases (27).
Germ cell tumors are the most common cancer in males between the ages of 15 and 35. They are divided into seminomatous and non-seminomatous subtypes and include embryonal carcinoma, yolk sac carcinoma, choriocarcinoma, and teratoma. Elevated serum hCG or hCG subunits may be present in both seminomatous and non-seminomatous types of germ cell tumors, whereas AFP may be elevated only in the non-seminomatous type. As a result of the increased hCG, acting analogously to LH to stimulate the Leydig cell LH receptor, testicular testosterone and estrogen (estrogen out of proportion to testosterone) production is increased, which, in turn, can cause gynecomastia. Although germ cell tumors generally arise in the testes, they can also originate extra-gonadally, specifically in the mediastinum. These extragonadal tumors also possess the capability of producing hCG, but they must be differentiated from a multitude of other tumors such as large cell carcinomas of the lung that can synthesize hCG or hCG subunits (28).
Some neoplasms that overproduce estrogens also possess aromatase overactivity. Sertoli cell tumors in boys with Peutz-Jegher syndrome, an autosomal dominant disease characterized by pigmented macules on the lips, gastrointestinal polyposis, and hormonally active tumors in males and females, for instance, have aromatase overactivity, resulting in gynecomastia, rapid growth and advanced bone age as presenting features (29, 30, 31). Feminizing Sertoli cell tumors with increased aromatase activity can also be seen in the Carney complex, an autosomal dominant disease characterized by cardiac myxomas, cutaneous pigmentation, adrenal nodules, and hypercortisolism. Other than sex-cord tumors, fibrolamellar hepatocellular carcinoma has also been shown to possess ectopic aromatase activity, causing severe gynecomastia in two boys (32, 33).
Furthermore, adrenal tumors can secrete excess dehydroepiandrosterone (DHEA), DHEA-sulfate (DHEAS), and androstenedione that can then be aromatized peripherally to estradiol. Some adrenal tumors may secret estrogen directly. Typically, feminizing adrenal tumors are large, aggressive and malignant (90).
Table 1.
Tumors Causing Gynecomastia
View in own window
| Tumor Type | Hormone Produced | Aromatase Overactivity |
|---|---|---|
| Leydig cell tumor | Testosterone, estrogen | |
| Sertoli cell tumor | Estrogen | + (in Peutz-Jegher Syndrome) + (in Carney complex) |
| Granulosa cell tumor | Estrogen | |
| Adrenal tumor | Estrogen, dehydroepiandrosterone (DHEA), dehydroepiandrosterone-sulfate (DHEA-S), and androstenedione which are converted in the periphery to estrogens. | |
| Gonadal germ cell tumor | hCG and β-hCG | |
| Extragonadal germ cell tumor (lung, gastric, renal cell and hepatocellular carcinoma | hCG and β-hCG (ectopic) |
NON-TUMOR CAUSES OF ESTROGEN EXCESS
Increased Aromatase Activity
Besides tumors, other conditions have been associated with excessive aromatization of testosterone and other androgens to estrogen, which results in gynecomastia. For instance, a familial form of gynecomastia has been discovered, in which affected family members have an elevation of extragonadal aromatase activity (34). Novel gain-of-function mutations in chromosome 15 have been reported to cause gynecomastia, possibly by forming cryptic promoters that lead to over expression of aromatase (35). More recently, polymorphism of the aromatase cytochrome P45019 (CYP19) has been found to be associated with gynecomastia (36). Obesity is thought to cause estrogen excess through increased aromatase activity in adipose tissue. Having said that, most obese men do not have elevated serum estrogen concentrations. Hyperthyroidism induces gynecomastia through several mechanisms, including increased aromatase activity (2).
Displacement of Estrogens from SHBG
Another cause of gynecomastia from estrogen excess includes steroid displacement from sex-hormone binding globulin (SHBG). SHBG binds androgens more avidly than estrogen. Thus, any condition or drug such as spironolactone that displaces steroids from SHBG, will more easily displace estrogen, allowing for higher circulating levels of estrogen. Drugs can cause gynecomastia by numerous mechanisms besides displacement from SHBG. These drugs and their mechanisms will be addressed in a subsequent section.
Decreased Testosterone and Androgen Resistance
Breast development requires the presence of estrogen. Androgens, on the other hand, oppose the estrogenic effects. Thus, equilibrium exists between estrogen and androgens in the adult male to prevent growth of breast tissue, whereby either an increase in estrogen or a decrease in androgen can tip the balance toward gynecomastia. Increased estrogen levels will increase glandular proliferation by several mechanisms. These include direct stimulation of glandular tissue and by suppressing LH, therefore decreasing testosterone secretion by the testes and exaggerating the already high estrogen to androgen ratio.
Besides increased estrogen production, decreased testosterone levels can cause an elevation in the estrogen to androgen ratio, producing gynecomastia. Primary hypogonadism, with its reduction in serum testosterone and increased serum LH levels increases aromatization of testosterone to estradiol and is associated with an increased estrogen to androgen ratio. Klinefelter syndrome occurs in 1 in 600-700 males and is caused by supernumerary X chromosomes (XXY or XXXY karyotype) and primary testicular failure and often prominent gynecomastia, due to decreased testosterone production, compensatory increased LH secretion, overstimulation of the Leydig cells and relative estrogen excess. In addition, any acquired testicular disease resulting in primary hypogonadism such as severe, postpubertal viral and bacterial orchitis, or scrotal trauma or radiation can promote gynecomastia by the same mechanisms (24). Lastly, enzyme deficiencies in the testosterone synthesis pathway from cholesterol also result in depressed testosterone levels and hence a relative increase in estrogen. Deficiency of 17-oxosteroid reductase, the enzyme that catalyzes the conversion of androstenedione to testosterone and estrone and estrone to estradiol, for example, will cause elevation in estrone and androstenedione, which is then further aromatized to estradiol (22).
Secondary hypogonadism results in low serum testosterone and unopposed estrogen effect from increased conversion of adrenal precursors to estrogens (24). Thus, patients with Kallmann syndrome, a form of congenital secondary hypogonadism with anosmia, also develop gynecomastia. In fact, androgen deficiency (hypogonadism) from whatever cause constitutes most cases of gynecomastia.
The androgen resistance syndromes, including complete and partial testicular feminization (e.g. Reifenstein's syndrome) are characterized by gynecomastia and varying degrees of pseudohermaphroditism. Kennedy disease, a neurodegenerative disease, is also associated with decreased effective testosterone due to a defective androgen receptor (2). The gynecomastia is the combined result of decreased androgen responsiveness at the breast level and increased estrogen levels as a result of elevated androgen precursors of estradiol and estrone. In these diseases the peripheral tissues, including the breast and pituitary, are less responsive to testosterone and other androgens. Androgen resistance at the pituitary results in elevated serum LH levels and increased circulating testosterone. The increased serum testosterone is then aromatized peripherally, promoting gynecomastia. Thus, gynecomastia is the result of increased estradiol levels that arise due to unopposed androgen unresponsiveness.
Other Diseases
Other disease states may also result in gynecomastia.
Men with end stage renal disease may have reduced testosterone, and elevated gonadotropins. This apparent primary testicular failure may then lead to increased breast development (13).
The gynecomastia of liver disease, particularly cirrhosis, does not have a clear etiology. Some have speculated that the gynecomastia is the result of estrogen overproduction, possibly secondary to increased extraglandular aromatization of androstenedione, which may have decreased hepatic clearance in cirrhotics. However, testosterone administration to cirrhotics causes a rise in estradiol, but decreases the prevalence of gynecomastia (5, 37, 38). Therefore, although the association of gynecomastia with liver disease is apparent, current data are conflicting and the mechanism remains unclear.
Thyrotoxicosis is associated with gynecomastia. Patients often have elevated estrogen levels that may result from a stimulatory effect of thyroid hormone on peripheral aromatase. Untreated thyrotoxicosis is often associated with high or high normal total testosterone, very high SHBG and low or low-normal free testosterone. Since SHBG binds testosterone more avidly than estradiol, there is a higher ratio of free estradiol to free testosterone. Thus, with normal testosterone and increased estrogen, there is an elevated free estrogen to testosterone ratio. In addition, LH is also increased, which may also stimulate testicular estrogen synthesis (39, 13).
Gynecomastia can also follow spinal cord disorders. Most patients with spinal cord disorders display depressed testosterone levels and, in fact, can develop testicular atrophy with resultant hypogonadism and infertility. Some have speculated that this may result from recurrent urinary tract infections, increased scrotal temperature, and a neuropathic bladder, which ultimately cause acquired primary testicular failure. The exact mechanism, however, remains elusive (40).
Refeeding gynecomastia refers to breast development in men recovering from a malnourished state (1). Although most cases regress within several months, the etiology of this phenomenon has not been fully elucidated.
HIV patients can also develop gynecomastia. There is a high incidence of androgen deficiency due to multifactorial causes, including primary and secondary hypogonadism and certain drugs used to treat HIV (e.g., efavirenz) (24).
Drugs
Around 20% of gynecomastia is caused by medications or exogenous chemicals (41). Some drugs may increase estrogen effect by several mechanisms: 1) they possess intrinsic estrogen-like properties, 2) they increase endogenous estrogen production, or 3) they supply an excess of an estrogen precursor (e.g. testosterone or androstenedione) which can be aromatized to estrogen. Examples of drugs that cause gynecomastia are listed in Tables 2 and 3. Contact with estrogen vaginal creams, for instance, can elevate circulating estrogen levels. Since some of the creams contain synthetic estrogens, they may not be detected by standard estrogenic qualitative assays. An estrogen-containing embalming cream has been reported to cause gynecomastia in morticians (42, 43). A topical estrogen spray, used for relief of menopausal hot flushes may lead to gynecomastia in children through skin contact (44). Recreational use of marijuana, heroin, methadone and amphetamines has also been associated with gynecomastia (45). Herbs containing phytoestrogen or Panax ginseng with estrogen-like structure (46) may also lead to gynecomastia. It has been suggested that digitalis causes gynecomastia due to its ability to bind to estrogen receptors (13, 47). The appearance of gynecomastia has been described in body builders and athletes after the administration of aromatizable androgens. The gynecomastia was presumably caused by an excess of circulating estrogens due to the conversion of androgens to estrogen by peripheral aromatase enzymes (48).
Table 2.
Drugs That May Induce Gynecomastia by Known or Proposed Mechanisms
View in own window
| Mechanism | Drugs |
|---|---|
| Estrogen-like, or binds to estrogen receptor | Estrogen vaginal cream Estrogen-containing embalming cream Delousing powder Digitalis Clomiphene Marijuana* |
| Stimulate estrogen synthesis | Gonadotropins Growth Hormone |
| Supply aromatizable estrogen precursors | Exogenous androgen Androgen precursors (i.e. androstenedione and DHEA) |
| Direct testicular damage | Busulfan Nitrosurea Vincristine Ethanol |
| Block testosterone synthesis | Ketoconazole Spironolactone Metronidazole Etomidate |
| Block androgen action | Flutamide Bicalutamide Finasteride Cyproterone Zanoterone Cimetidine Ranitidine* Spironolactone |
| Displace estrogen from SHBG | Spironolactone Ethanol |
Table 3.
Drugs That Cause Gynecomastia by Uncertain Mechanisms
View in own window
| Cardiac and antihypertensive medications: Calcium channel blockers (verapamil, nifedipine, diltiazem) ACE Inhibitors*(captopril, enalapril) Alpha-blockers* Amiodarone Methyldopa Reserpine Nitrates |
| Psychoactive drugs: Neuroleptics Anxiolytic agents* e.g. Diazepam Phenytoin Tricyclic antidepressants Haloperidol Atypical antipsychotic agents |
| Drugs for infectious diseases: Antiretroviral therapy for HIV/AIDS (e.g. efavirenz) Isoniazid Ethionamide Griseofulvin Minocycline |
| Drugs of Abuse: Amphetamines Heroin Methadone |
| Others: Theophylline Omeprazole Auranofin Diethylpropion Domperidone Penicillamine Sulindac Heparin Methotrexate Statin* |
Drugs and chemicals that cause decreased testosterone levels either by causing direct testicular damage, by blocking testosterone synthesis, or by blocking androgen action can also produce gynecomastia. For instance, phenothrin, a chemical component in delousing agents, possessing antiandrogenic activity, has been attributed as the cause of an epidemic of gynecomastia among Haitian refugees in US detention centers in 1981 and 1982 (49). Chemotherapeutic drugs, such as alkylating agents, cause Leydig cell and germ cell damage, resulting in primary hypogonadism. Flutamide, an anti-androgen used as treatment for prostate cancer, blocks androgen action in peripheral tissues, while cimetidine blocks androgen receptors. Ketoconazole, on the other hand, can inhibit steroidogenic enzymes required for testosterone synthesis. 5α-reductase inhibitors, finasteride and dutasteride that reduce the conversion of testosterone to dihydrotestosterone may cause gynecomastia (50). Spironolactone causes gynecomastia by several mechanisms. Like ketoconazole, it can block androgen production by inhibiting enzymes in the testosterone synthetic pathway (i.e. 17a hydroxylase and 17-20-desmolase), but it can also block receptor-binding of testosterone and dihydrotestosterone (51). In addition to decreasing testosterone levels and biologic effects, spironolactone also displaces estradiol from SHBG, increasing free estrogen levels. Of note, the antiandrogenic property of spironolactone has been used in gender identity disorder (from male to female) and spironolactone is considered to be a cost-saving medication (52). On the other hand, eplerenone is more specific for mineralocorticoid receptor and less associated with antiandrogenic effects such as gynecomastia (53). Switching from spironolactone to eplerenone may reverse painful gynecomastia induced by spironolactone in cirrhotics (54). Ethanol increases the estrogen to androgen ratio and induces gynecomastia by multiple mechanisms as well. Firstly, it is associated with increased SHBG, which decreases free testosterone levels. Secondly, it increases hepatic clearance of testosterone, and thirdly, it has a direct toxic effect on the testes themselves (24). Unfortunately, besides the drugs stated, a multitude of others cause gynecomastia by unknown mechanisms (92) (Table 3).
MALE BREAST CANCER
Male breast cancer is rare and comprises only 0.2 percent of all male cancers. The overall prevalence of invasive carcinomas was 0.11% and of in situ carcinomas was 0.18% in surgically excised breast specimens with the diagnosis of gynecomastia (55). Despite these low figures, men with gynecomastia, especially elderly, worry about breast cancer and often seek medical advice, making this sort of consultation rather common in primary health care (56). Male breast cancer, though uncommon, has been associated with gynecomastia and necessitates inclusion in the differential diagnosis. Men with Klinefelter syndrome have a 20- to 50-fold increased risk of breast cancer. Other risks include hyperestrogenic conditions like obesity, alcohol, exogenous estrogen exposure, and testicular disorders. It is unclear if these are specific risks for breast cancer are linked to the stimulatory process responsible for gynecomastia (57). High ambient temperature, exhaust emissions, radiation to chest, and liver damage are also risk factors for male breast cancer (58). Family history should always be explored. In particular, a family history of BRCA2 positive breast cancer significantly increases the risk of male breast cancer in carriers of mutation (59).
PATIENT EVALUATION
History and Physical Examination
At presentation, all patients require a thorough history and physical exam. Particular attention should be given to medications, drug and alcohol abuse, as well as other chemical exposures. Symptoms of underlying systemic illness, such as hyperthyroidism, liver disease, or renal failure should be sought. Furthermore, the clinician must recall neoplasm as a possible etiology and should establish the duration and timing of breast development. Rapid breast growth that has occurred recently is more concerning than chronic gynecomastia. Additionally, the clinician should inquire about fertility, erectile dysfunction and libido to rule out hypogonadism, either primary or secondary, as a potential cause.
In our experience, the breast examination is best performed with the patient supine and with the examiner palpating from the periphery to the areola. When firmness is noted the glandular mass should be measured in diameter. Clinically, gynecomastia is diagnosed by finding subareolar breast tissue of 2 cm in diameter or greater. Malignancy is suspected if an immobile firm mass is found on physical examination. Skin dimpling, nipple retraction or discharge, and axillary lymphadenopathy further support malignancy as a possible diagnosis. Tenderness may be present in patients with gynecomastia of less than 6 months duration, but it is unusual in patients with breast cancer.
A thorough testicular exam is essential. When clinical exam suggests a testicular mass or when serum hCG is elevated, testicular ultra-sound (USG) is warranted. Bilaterally small testes imply testicular failure, while asymmetric testes or a testicular mass suggest the possibility of neoplasm. Visual field impairment may suggest pituitary disease. Physical findings of underlying systemic conditions such as thyrotoxicosis, HIV disease, liver, or kidney failure should also be assessed. As obesity is often associated with gynecomastia, body mass index should be documented (56).
Laboratory Evaluation
All patients who present with gynecomastia should have serum testosterone, estradiol, LH and hCG measured (93) (using an assay that detects all forms of hCG) (Fig 2). Further testing should be tailored according to the history, physical examination and the results of these initial tests. An elevated beta-HCG or a markedly elevated serum estradiol suggests neoplasm and a testicular ultrasound is warranted to identify a testicular tumor, keeping in mind, however, other non-testicular tumors can also secrete hCG. A low testosterone level, with an elevated LH and normal to high estrogen level indicates primary hypogonadism. If the history suggests Klinefelter syndrome, then a karyotype should be performed for definitive diagnosis. Low testosterone, low LH and normal estradiol levels indicate secondary hypogonadism, and hypothalamic or pituitary causes should be sought. If testosterone, LH and estradiol levels are all elevated, then the diagnosis of androgen resistance should be considered. Liver, kidney and thyroid function should be assessed if the physical examination suggests liver failure, kidney failure, or hyperthyroidism, respectively. A chest x-ray should be done if a lung or mediastinal lesion is suspected. Furthermore, if examination of breast tissue suggests malignancy, a biopsy should be performed. This is of particular importance in patients with Klinefelter syndrome, who have an increased risk of breast cancer. On the other hand, if the examination finding is compatible with breast abscess, then fine needle aspiration for microscopy, acid-fast bacilli and culture is warranted (60).
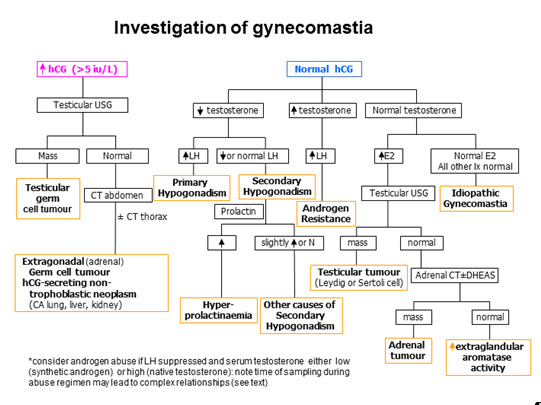
Figure 2.
Algorithm for Investigation of Gynecomastia
TREATMENT
Treatment of the underlying endocrinologic or systemic disease that has caused gynecomastia is appropriate when possible. Testicular tumors, such as Leydig cell, Sertoli cell or granulosa cell tumors should be surgically removed. In addition to surgery, germ cell tumors are further managed with chemotherapy involving cisplatin, bleomycin and either vinblastine or etoposide (25, 26). Should underlying thyrotoxicosis, renal or hepatic failure be discovered, appropriate therapy should be initiated. Medications that cause gynecomastia should also be discontinued whenever possible based on their role in management of the underlying condition. The improvement should be apparent within a month after discontinuation of the culprit drug (61). If the gynecomastia has been present for more than six months, regression is unlikely because of the presence of less reversible fibrotic tissues (62). Of course, if a breast biopsy indicates malignancy, then mastectomy should be performed.
If no pathologic etiology is detected, then appropriate treatment is close observation. A careful breast exam should be done initially every 3-6 months until the gynecomastia regresses or stabilizes, after which a breast exam can be performed yearly. It is important to remember that most cases of pubertal gynecomastia may resolve spontaneously within one to two years, around 20% of patients have residual gynecomastia by the age of 20 (63). An information sheet about gynecomastia is available for those patients who are interested to know more about their conditions (64).
Medical Treatment
If the gynecomastia is severe, does not resolve, of recent onset (less than 6 months), and does not have a treatable underlying cause, some medical therapies may be attempted. There are 3 classes of medical treatment for gynecomastia: androgens (testosterone, dihydrotestosterone, danazol), anti-estrogens (clomiphene citrate, tamoxifen), and aromatase inhibitors such as letrozole and anastrazole.
Once gynecomastia is established, testosterone treatment of hypogonadal men with gynecomastia often fails to produce breast regression. Unfortunately, testosterone treatment may actually produce the side effect of gynecomastia by being aromatized to estradiol. Thus, although testosterone is used to treat hypogonadism, its use to specifically counteract gynecomastia is limited (65). Dihydrotestosterone, a non-aromatizable androgen, has been used in patients with prolonged pubertal gynecomastia with good response rates (66). Danazol, a weak androgen that inhibits gonadotropin secretion, resulting in decreased serum testosterone levels, has been studied in a prospective placebo-controlled trial, whereby gynecomastia resolved in 23 percent of the patients, as opposed to 12 percent of the patients on placebo (67). The dose used for gynecomastia is 200 mg orally twice daily. Unfortunately, undesirable side effects including edema, acne, and cramps have limited its use (24).
Investigators have reported a 64 percent response rate with 100 mg/day of clomiphene citrate, a weak estrogen and moderate anti-estrogen (68). Lower doses of clomiphene have shown varied results, indicating that higher doses may need to be administered, if clomiphene is to be attempted. Tamoxifen, also an anti-estrogen, has been studied in 2 randomized, double-blind studies in which a statistically significant regression in breast size was achieved, although complete regression was not documented (69). One study compared tamoxifen with danazol in the treatment of gynecomastia. Although patients taking tamoxifen had a greater response with complete resolution in 78 percent of patients treated with tamoxifen, as compared to only a 40 percent response in the danazol-treated group, the relapse rate was higher for the tamoxifen group (70). Another prospective cohort study found that 90% of patients taking tamoxifen had successful resolution of their symptoms (89). Although there is a chance of recurrence with cessation of therapy, tamoxifen, due to relatively lower side effect profile and high efficacy, may be a more reasonable choice when compared to the other therapies. If used, tamoxifen should be given at a dose of 10 mg twice or 20 mg daily a day for 3-6 months (24). Responders usually improve with reduced pain within 1 month. Another anti-estrogen, raloxifene, has also been used in the treatment of pubertal gynecomastia but its efficacy needs to be evaluated in randomized prospective studies (71).
An aromatase inhibitor, testolactone, has also been studied in an uncontrolled trial with promising effects (72). Further studies must be performed on this drug before any recommendations can be established on its usefulness in the treatment of gynecomastia. Newer aromatase inhibitors such as anastrozole and letrozole may have therapeutic potential (73, 74), but randomized, double-blind, placebo-controlled trials have not confirmed its efficacy. In a study involving patients receiving bicalutamide therapy for prostate cancer, only tamoxifen, but not anastrozole, significantly reduced the incidence of gynecomastia/breast pain when used prophylactically and therapeutically (75, 76). In another study with pubertal gynecomastia, no significant difference was demonstrated between the anastrozole and placebo groups in patients suffering from pubertal gynecomastia (77). The use of aromatase inhibitors is notorious for accelerated bone loss in women, but it is uncertain whether the extent of bone loss is similar in adult men. (91).
From various series, many patients with gynecomastia show no significant improvement after medical treatment. This may be related to the stage of disease at which medical treatment is initiated. It has been suggested that patient with long history of gynecomastia, in which the breast tissue becomes fibrotic and tends to be resistant to medical treatment (56, 63).
Surgical Treatment
When medical therapy is ineffective, particularly in cases of longstanding gynecomastia, or when the gynecomastia interferes with the patient's activities of daily living, or when there is suspicion of malignancy of breast, then surgical therapy is appropriate. On the other hand, surgical treatment should be postponed in pubertal gynecomastia, preferably until after completion of puberty, so as to minimize the chance of recurrent gynecomastia after surgery (62). Surgery should also be deferred until the underlying cause of gynecomastia has resolved or been treated. Surgical treatment includes removal of glandular tissue coupled with liposuction, if needed, preferably with individualized approach (78, 79). Nowadays, minimally invasive surgery is available and it may be associated with few complications and prompt recovery (80). Of note if malignancy is suspected, histological examination is mandatory (56). Uses of delicate cosmetic surgical techniques are warranted to prevent unsightly scarring.
PREVENTION OF GYNECOMASTIA IN MEN WITH PROSTATE CANCER
Because androgen deprivation is one of the commonly used treatment modalities for advanced prostate cancer, its possible role in the development of gynecomastia is of particular concern to clinicians. Up to 80% of patients receiving non-steroidal anti-androgen therapy may develop gynecomastia, usually 6-9 months after hormonal treatment. Some patients may have painful and disfiguring gynecomastia (81). Several preventive strategies have been proposed: Tamoxifen has demonstrated its efficacy versus radiotherapy in preventing gynecomastia in patients receiving bicalutamide (Casodex) for prostate cancer in a randomized controlled trial (82). Boccardo et al showed 10% patients in the tamoxifen group (20 mg daily dose) developed gynecomastia, whereas 51% in the anastrozole group and 73% in placebo group had gynecomastia over a period of 48 weeks (74). Fradet et al showed tamoxifen reduced the incidence of gynecomastia in patients with prostate cancer receiving bicalutamide in dose dependent manner (83). Likewise, it has been shown that low dose tamoxifen (20 mg/week) is inferior to the daily regimen (20mg/day) in terms of the prevention and treatment of gynecomastia (84). Current data suggests tamoxifen 10-20mg per day is the optimum dose required for prophylaxis of gynecomastia in patients with prostate cancer receiving androgen deprivation therapy (84, 83, 85). Low dose prophylactic irradiation has been variably reported to reduce the rate of gynecomastia in men receiving estrogens or anti-androgens for prostate cancer (86, 11, 87). Some papers suggest that the new generation of anti-androgen therapy, such as abiraterone acetate, may be associated with less gynecomastia (88); further studies are required to confirm these results.
REFERENCES
- 1.
-
Franz A, Wilson J: Williams Textbook of Endocrinology ninth edition, 877-885, 1998.
- 2.
-
Santen R: Endocrinology fourth edition vol. 3: 2335-2341, 2001.
- 3.
-
Bocchinfuso WP, Korach KS. Mammary Gland Development and Tumorigenesis in Estrogen Receptor Knockout Mice. Journal of Mammary Gland Biology and Neoplasia. 1997;90:323–334. [PubMed: 10935020]
- 4.
-
Lubahn DB, Moyer JS, Golding TS. Alteration of Reproductive Function but not Prenatal Sexual Development after Insertional Disruption of the Mouse Estrogen Receptor Gene. Proc Soc Natl Acad Sci USA. 1993;90:11162–11166. [PMC free article: PMC47942] [PubMed: 8248223]
- 5.
-
Edman DC, Hemsell DL, Brenner PF. Extraglandular Estrogen Formation in Subjects with Cirrhosis. Gastroenterology. 1975;69:819.
- 6.
-
Kleinberg DL, Feldman M, Ruan W. IGF-1: An Essential Factor in Terminal End Bud Formation and Ductal Morphogenesis. Journal of Mammary Gland Biology and Neoplasia. 2000;5(1):7–17. [PubMed: 10791764]
- 7.
-
Ruan W, Kleinberg DL. Insulin-like Growth Factor I is Essential for Terminal End Bud Formation and Ductal Morphogenesis during Mammary. Development. Endocrinology. 1999;140(11):5075–81. [PubMed: 10537134]
- 8.
-
Walden PD, Ruan W, Feldman M, Kleinberg DL. Evidence that the Mammary Fat Pad Mediated the Action of Growth Hormone in Mammary Gland Development. Endocrinology. 1998;139(2):659–62. [PubMed: 9449638]
- 9.
-
Mieritz MG, Sorensen K, Aksglaede L, et al. Elevated serum IGF-I, but unaltered sex steroid levels, in healthy boys with pubertal gynaecomastia. Clin Endocrinol (Oxf). 2014 May;80(5):691–8. [PubMed: 24033660]
- 10.
-
Mol JA, Van Garderen E, Rutteman GR, Rijnberk A. New Insights in the Molecular Mechanism of Progestin-induced Proliferation of Mammary Epithelium: Induction of the Local Biosynthesis of Growth Hormone in the Mammary Gland of Dogs, Cats, and Humans. Journal of Steroid Biochemistry and Molecular Biology. 1996;57(1-2):67–71. [PubMed: 8645618]
- 11.
-
Ozen H, Akyol F, Toktas G, Eskicorapci S, Unluer E, Kuyumcuoglu U, Abay E, Cureklibatur I, Sengoz M, Yalcin V, Akpinar H, Zorlu F, Sengor F, Karaman I. Is prophylactic breast radiotherapy necessary in all patients with prostate cancer and gynecomastia and/or breast pain? J Urol. 2010 Aug;184(2):519–24. [PubMed: 20620411]
- 12.
-
Sasano H. Kimura m, Shizawa s, Kimura N, Nagua H, Aromatase and Steroid Receptors in Gynecomastia and Male Breast Carcinoma: an Immunohistochemical Study. Journal of Clinical Endocrinology and Metabolism. 1996;81(8):3063–7. [PubMed: 8768875]
- 13.
-
Glass AR. Gynecomastia. Endocrinology and Metabolism Clinics of North America. 1994;23(4):825–837. [PubMed: 7705322]
- 14.
-
LeProvost F, Leroux C, Martin P, Gaye P. Djiane, J, Prolactin Gene Expression in Ovine and Caprine Mammary Gland. Neuroendocrinology. 1994;60:305–313. [PubMed: 7969789]
- 15.
-
Steinmetz R, Grant A, Malven P. Transcription of Prolactin Gene in Milk Secretory Cells of the Rat Mammary Gland. Journal of Endocrinology. 1993;36:305–313. [PubMed: 8459191]
- 16.
-
Carlson HE, Kane P, Lei ZM, et al. Presence of luteinizing hormone/human chorionic gonadotropin receptors in male breast tissues. J Clin Endocrinol Metab. 2004;89(8):4119–23. [PubMed: 15292356]
- 17.
-
Gill K, Kirma N, Tekmal RR. Overexpression of Aromatase in Transgenic Male Mice Results in the Induction of Gynecomastia and other Biochemical Changes in Mammary Gland. Journal of Steroid Biochemistry and Molecular Biology. 2001;77(1):13–18. [PubMed: 11358670]
- 18.
-
Li X, Warri A, Makela S, Ahonen T, Streng T, Santti R, Poutanen M. Mammary gland development in transgenic male mice expressing human P450 aromatase. Endocrinology. 2002;143(10):4074–83. [PubMed: 12239119]
- 19.
-
Moore DC, Schlaepfer LP, Sizonenko PC. Hormonal Changes During Puberty: Transient Pubertal Gynecomastia; Abnormal Androgen-Estrogen Ratios. Journal of Clinical Endocrinology and Metabolism. 1984;58:492–499. [PubMed: 6693546]
- 20.
-
Mahoney CP. Adolescent Gynecomastia. Differential Diagnosis and Management. Pediatric Clinics of North America. 1990;37(6):1389–1404. [PubMed: 2259545]
- 21.
-
Niewoehner CB, Nuttall FQ. Gynecomastia in Hospitalized Male Population. American Journal of Medicine. 1984;77:633–638. [PubMed: 6486139]
- 22.
-
Braunstein GD. Aromatase and Gynecomastia. Endocrine-Related Cancer. 1999;6:315–324. [PubMed: 10731125]
- 23.
-
Ikard RW, Vavra D, Forbes RC, Richman JC, Roumie CL. Management of senescent gynecomastia in the Veterans Health Administration. Breast J. 2011 Mar;17(2):160–6. [PubMed: 21410583]
- 24.
-
Mathur R. Braunstein: Gynecomastia: Pathomechanisms and Treatment Strategies. Hormone Research. 1997;48:95–102. [PubMed: 11546925]
- 25.
-
Gana BM. Leydig Cell Tumor. British Journal of Urology. 1995;75(5):676–8. [PubMed: 7613811]
- 26.
-
Richie J: Campbell's Urology 7th Edition, 2439-2443, 1998.
- 27.
-
Hanson JA, Ambaye AB. Adult testicular granulosa cell tumor: a review of the literature for clinicopathologic predictors of malignancy. Arch Pathol Lab Med. 2011 Jan;135(1):143–6. [PubMed: 21204721]
- 28.
-
Moran CA, Suster S. Primary Mediastinal Choriocarcinoma: A Clinicopathologic and Immunohistochemical Study of Eight Cases. American Journal of Surgical Pathology. 1997;21(9):1007–1012. [PubMed: 9298876]
- 29.
-
Coen P, Kulin H, Ballantine T. Zaino r, Frauenhoffer E, Boal D, Inkster S, Brodie A, Santen R: An Aromatase-Producing Sex-cord Tumor Resulting in Prepubertal Gynecomastia. New England Journal of Medicine. 1991;324(5):317–22. [PubMed: 1986290]
- 30.
-
Hertl MC, Wiebel J, Schafer H, Willig HP, Lambrecht W. Feminizing Sertoli Cell Tumors Associated with Peutz-Jeghers Syndrome: An Increasingly Recognized Cause of Prepubertal Gynecomastia. Plastic Reconstructive Surgery. 1998;102(4):1151–57. [PubMed: 9734436]
- 31.
-
Young S, Gooneratne S. Straus FH 2nd, Zeller WP, Bulun SE, Rosenthal IM: Feminizing Sertoli Cell Tumors in Boys with Peutz-Jehgers Syndrome. American Journal of Surgical Pathology. 1995;19(1):50–58. [PubMed: 7802138]
- 32.
-
Agarwal VR, Takayama K, Van Wyk JJ, Sasano H, Simpson ER, Bulun SE. Molecular Basis of Severe Gynecomastia Associated with Aromatase Expression in a Fibrolamellar Hepatocellular Carcinoma. Journal of Clinical Endocrinology and Metab. 1988;83(5):1797–1800. [PubMed: 9589695]
- 33.
-
Muramori K, Taguchi S, Taguchi T, Kohashi K, Furuya K, Tokuda K, Ishii E. High Aromatase Activity and Overexpression of Epidermal Growth Factor Receptor in Fibrolamellar Hepatocellular Carcinoma in a Child. J Pediatr Hematol Oncol. 2011;(May):5. [PubMed: 21552145]
- 34.
-
Berkovitz GD. Guerami, Brown TR, MacDonald PC Migeon CJ: Familial Gynecomastia with Increased Extraglandular Aromatization of Plasma Carbon 19-Steroids. Journal of Clinical Investigation. 1985;75:1763–1769. [PMC free article: PMC425530] [PubMed: 3924954]
- 35.
-
Shozu M, Sebastian S, Takayama K, Hsu WT, Schultz RA, Neely K, Bryant M, Bulun SE. Estrogen excess associated with novel gain-of-function mutations affecting the aromatase gene. N Engl J Med 8;348(19):1855-65, May, 2003. [PubMed: 12736278]
- 36.
-
Czajka-Oraniec I, Zgliczynski W, Kurylowicz A, Mikula M, Ostrowski J. Association between gynecomastia and aromatase (CYP19) polymorphisms. Eur J Endocrinol. 2008 May;158(5):721–7. [PubMed: 18426832]
- 37.
-
Bahnsen M, Gluud C, Johnsen SG. Pituitary-testicular Function in Patients with Alcoholic Cirrhosis of the Liver. European Journal of Clinical Investigation. 1981;11:473–479. [PubMed: 6460638]
- 38.
-
Olivo J, Gordon GG, Raifi F. Estrogen Metabolism in Hyperthyroidism and in Cirrhosis of the Liver. Steroids. 1975;26:47–56. [PubMed: 1166483]
- 39.
-
Chan WB, Yeung VT, Chow CC, So WY, Cockram CS. Gynaecomastia as a Presenting Feature of Thyrotoxicosis. Postgraduate Medical Journal. 1999;75(882):229–231. [PMC free article: PMC1741202] [PubMed: 10715765]
- 40.
-
Herito RJ, Dankner R, Berezin M, Zeilig G, Ohry A. Gynecomastia Following Spinal Cord Disorder. Archives of Physical Medicine and Rehabilitation. 1997;78(5):534–537. [PubMed: 9161376]
- 41.
-
Bowman JD, Kim H, Bustamante JJ. Drug-induced gynecomastia. Pharmacotherapy. 2012 Dec;32(12):1123–40. [PubMed: 23165798]
- 42.
-
Bhat N, Rosato E, Gupta P. gynecomastia in a mortician: A case report. Acta Cytol. 1990;34:31. [PubMed: 2296837]
- 43.
-
Finkelstein J, McCully W, MacLaughlin D, et al. The mortician's mystery: Gynecomastia and reversible hypogonadotropic hypogonadism in an embalmer. N Eng J Med. 1988;319:961. [PubMed: 3352686]
- 44.
-
Voelker R. Estrogen spray poses risks to children, pets through contact with treated skin. JAMA. 2010 Sep 1;304(9):953. [PubMed: 20810368]
- 45.
-
Nordt CA, DiVasta AD. Gynecomastia in adolescents. Curr Opin Pediatr. 2008 Aug;20(4):375–82. [PubMed: 18622190]
- 46.
-
Kakisaka Y, Ohara T, Tozawa H. Panax ginseng: a newly identified cause of gynecomastia. Tohoku J Exp Med. 2012;228(2):143–5. [PubMed: 23006978]
- 47.
-
Rifka SM, Pita JC, Vigersky RA, et al. Interaction of digitalis and spironolactone with human sex steroid receptors. J Clin Endocrinol Metab. 1977;46:228–244. [PubMed: 86546]
- 48.
-
Calzada L, Torres-Calleja JM, Martinez N. Measurement of Androgen and Estrogen Receptors in Breast Tissue from Subjects with Anabolic Steroid-Dependent Gynecomastia. Life Sciences. 69(2110):1465–1479. [PubMed: 11554608]
- 49.
-
Brody SA, Loriaux DL. Epidemic of gynecomastia among Haitian refugees: exposure to an environmental antiandrogen. Endocr Pract 9(5):370-5, Set-Oct, 2003. [PubMed: 14583418]
- 50.
-
Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse Side Effects of 5α-Reductase Inhibitors Therapy: Persistent Diminished Libido and Erectile Dysfunction and Depression in a Subset of Patients. J Sex Med. 2011 Mar;8(3):872–84. [PubMed: 21176115]
- 51.
-
Thompson DF, Carter J. Drug-induced gynecomastia. Pharmacotherapy. 1993;13(1):37–45. [PubMed: 8094898]
- 52.
-
Spack NP. Management of transgenderism. JAMA. 2013 Feb 6;309(5):478–84. [PubMed: 23385274]
- 53.
-
Parthasarathy HK, Ménard J, White WB, Young WF Jr, Williams GH, Williams B, Ruilope LM, McInnes GT, Connell JM, Macdonald TM. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens. 2011 May;29(5):980–990. [PubMed: 21451421]
- 54.
-
Dimitriadis G, Papadopoulos V, Mimidis K. Eplerenone reverses spironolactone-induced painful gynaecomastia in cirrhotics. Hepatol Int. 2011 Jun;5(2):738–9. [PMC free article: PMC3090552] [PubMed: 21484105]
- 55.
-
Lapid O, Jolink F, Meijer SL. Pathological findings in gynecomastia: analysis of 5113 breasts. Ann Plast Surg. 2015 Feb;74(2):163–6. [PubMed: 23788148]
- 56.
- 57.
-
Hsing A, McLaughlin J. Cocco p, Chen H, Fraumeni JF: Risk factors for male breast cancer. Cancer Causes and Control. 1998;9:269–275. [PubMed: 9684707]
- 58.
-
Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006;367(9510):595–604. [PubMed: 16488803]
- 59.
-
Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010 Oct;47(10):710–1. [PubMed: 20587410]
- 60.
-
Koh J, Tee A. Images in clinical medicine. Tuberculous abscess manifesting as unilateral gynecomastia. N Engl J Med. 2009 Dec 3;361(23):2270. [PubMed: 19955527]
- 61.
-
Braunstein GD. Clinical practice. Gynecomastia. N Engl J Med. 2007 Sep 20;357(12):1229–37. [PubMed: 17881754]
- 62.
-
Carlson HE. Approach to the patient with gynecomastia. J Clin Endocrinol Metab. 2011 Jan;96(1):15–21. [PubMed: 21209041]
- 63.
-
Singh Narula H, Carlson HE. Gynaecomastia. Endocrinol Metab Clin North Am. 2007;48:497–519. [PubMed: 17543732]
- 64.
-
Hormone Foundation. Patient information page. Gynecomastia. J Clin Endocrinol Metab. 2011 Jan;96(1):0. [PubMed: 21260969]
- 65.
-
Treves N. Gynecomastia: the origins of mammary swelling in the male: and analysis of 406 patients with breast hypertrophy, 525 with testicular tumors, and 13 with adrenal neoplasms. Cancer. 1958;11:1083–102. [PubMed: 13608407]
- 66.
-
Kuhn JM, Roca R, Laudat MH, et al. Studies on the treatment of idiopathic gynecomastia with percutaneous dihydrotestosterone. Clin Endo. 1983;19:513–20. [PubMed: 6354523]
- 67.
-
Jones DJ, Holt SD, Surtees P, et al. A comparison of danazol and placebo in the treatment of adult idiopathic gynaecomastia: results of a prospective study in 55 patients. Ann R Coll Surg Engl. 1990;72:296–8. [PMC free article: PMC2499206] [PubMed: 2221763]
- 68.
-
Leroith D, Sobel R, Glick SM. The effect of clomiphene citrate on pubertal gynaecomastia. Acta Endocrinol. 1980;95:177–80. (copenh) [PubMed: 6776752]
- 69.
-
Alagaratnam TT. Idiopathic gynecomastia treated with tamoxifen; a preliminary report. Clin Ther. 1987;9:483–7. [PubMed: 3664552]
- 70.
-
Ting AC, Chow LW. Leung Yf: Comparison of tamoxifen with danazol in the management of idiopathic gynecomastia. Am Surg. 2000;66(1):38–40. [PubMed: 10651345]
- 71.
-
Lawrence SE, Faught KA, Vethamuthu J, Lawson ML. Beneficial effects of raloxifene and tamoxifen in the treatment of pubertal gynecomastia. J Pediatr. 2004;145(1):71–6. [PubMed: 15238910]
- 72.
-
Zachmann M, Eiholzer U, Muritano M, et al: Treatment of pubertal gynaecomastia with testolactone. Acta Endocrinol supple (copenh) 279:218-26, 1986. [PubMed: 3535334]
- 73.
-
Miller WR, Jackson J. The therapeutic potential of aromatase inhibitors. Expert Opin Investig Drugs. 2003 Mar;12(3):337–51. [PubMed: 12605559]
- 74.
-
Riepe FG, Baus I, Wiest S, et al: Treatment of Pubertal Gynecomastia with the Specific Aromatase Inhibitor Anastrozole. Horm Res 20;62(3):113-118, 2004. [PubMed: 15273427]
- 75.
-
Boccardo F, Rubagotti A, Battaglia M, et al: Evaluation of tamoxifen and anastrozole in the prevention of gynecomastia and breast pain induced by bicalutamide monotherapy of prostate cancer. J Clin Oncol 1;23(4):808-15, 2005. [PubMed: 15681525]
- 76.
-
Saltzstein D, Sieber P, Morris T, Gallo J. Prevention and management of bicalutamide-induced gynecomastia and breast pain: randomized endocrinologic and clinical studies with tamoxifen and anastrozole. Prostate Cancer Prostatic Dis. 2005;8(1):75–83. [PubMed: 15685254]
- 77.
-
Plourde PV, Reiter EO, Jou HC, Desrochers PE, Rubin SD, Bercu BB, Diamond FB Jr, Backeljauw PF. Safety and efficacy of anastrozole for the treatment of pubertal gynecomastia: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89(9):4428–33. [PubMed: 15356042]
- 78.
-
Fischer S, Hirsch T, Hirche C, et al. Surgical treatment of primary gynecomastia in children and adolescents. Pediatr Surg Int. 2014 Jun;30(6):641–7. [PubMed: 24763713]
- 79.
-
Hammond DC. Surgical correction of gynecomastia. Plast Reconstr Surg. 2009 Jul;124(1) Suppl:61e–68e. [PubMed: 19568140]
- 80.
-
Jarrar G, Peel A, Fahmy R, Deol H, Salih V, Mostafa A. Single incision endoscopic surgery for gynaecomastia. J Plast Reconstr Aesthet Surg. 2011 May 12; [PubMed: 21570372]
- 81.
-
Michalopoulos NV, Keshtgar MR. Images in clinical medicine. Gynecomastia induced by prostate-cancer treatment. N Engl J Med. 2012 Oct 11;367(15):1449. [PubMed: 23050528]
- 82.
-
Perdonà S, Autorino R, De Placido S, D'Armiento M, Gallo A, Damiano R, Pingitore D, Gallo L, De Sio M, Bianco AR, Di Lorenzo G. Efficacy of tamoxifen and radiotherapy for prevention and treatment of gynaecomastia and breast pain caused by bicalutamide in prostate cancer: a randomised controlled trial. Lancet Oncol. 2005 May;6(5):295–300. [PubMed: 15863377]
- 83.
-
Fradet Y, Egerdie B, Andersen M, Tammela TL, Nachabe M, Armstrong J, Morris T, Navani S. Tamoxifen as prophylaxis for prevention of gynaecomastia and breast pain associated with bicalutamide 150 mg monotherapy in patients with prostate cancer: a randomised, placebo-controlled, dose-response study. Eur Urol. 2007 Jul;52(1):106–14. [PubMed: 17270340]
- 84.
-
Bedognetti D, Rubagotti A, Conti G, Francesca F, De Cobelli O, Canclini L, Gallucci M, Aragona F, Di Tonno P, Cortellini P, Martorana G, Lapini A, Boccardo F. An open, randomised, multicentre, phase 3 trial comparing the efficacy of two tamoxifen schedules in preventing gynaecomastia induced by bicalutamide monotherapy in prostate cancer patients. Eur Urol. 2010 Feb;57(2):238–45. [PubMed: 19481335]
- 85.
-
Serretta V, Altieri V, Morgia G, et al. A randomized trial comparing tamoxifen therapy vs. tamoxifen prophylaxis in bicalutamide-induced gynecomastia. Clin Genitourin Cancer. 2012 Sep;10(3):174–9. [PubMed: 22502790]
- 86.
-
Chou JL, Easley JD, Feldmeier JJ, Rauth VA, Pomeroy TC. Effective radiotherapy in palliating mammalgia associated with gynecomastia after DES therapy. Int J Radiat Oncol Biol Phys. 1988;15(3):749–51. [PubMed: 2458332]
- 87.
-
Widmark A, Fossa SD, Lundmo P, Damber JE, Vaage S, Damber L, Wiklund F, Klepp O. Does prophylactic breast irradiation prevent antiandrogen-induced gynecomastia? Evaluation of 253 patients in the randomized Scandinavian trial SPCG-7/SFUO-3. J Urol. 2003;170(1):320. [PubMed: 12559286]
- 88.
-
Alesini D, Iacovelli R, Palazzo A, et al. Multimodality treatment of gynecomastia in patients receiving antiandrogen therapy for prostate cancer in the era of abiraterone acetate and new antiandrogen molecules. Oncology. 2013;84(2):92–9. [PubMed: 23128186]
- 89.
-
Mannu GS, Sudul M, Bettencourt-Silva JH, Tsoti SM, Cunnick G, Ahmed SF. Role of tamoxifen in idiopathic gynecomastia: A 10-year prospective cohort study. Breast J. 2018 Nov;24(6):1043–1045. [PubMed: 30079473]
- 90.
-
Ali SN, Jayasena CN, Sam AH. Which patients with gynaecomastia require more detailed investigation? Clin Endocrinol (Oxf). 2018 Mar;88(3):360–363. [PubMed: 29193251]
- 91.
-
Tan RB, Guay AT, Hellstrom WJ. Clinical use of aromatase inhibitors in adult males. Sex Med Rev. 2014 Apr;2(2):79–90. [PubMed: 27784593]
- 92.
-
He B, Carleton B, Etminan M. Risk of Gynecomastia with Users of Proton Pump Inhibitors. Pharmacotherapy. 2019 Mar 13; [PubMed: 30865318] [CrossRef]
- 93.
-
Gronowski AM. Clinical assays for human chorionic gonadotropin: what should we measure and how? Clin Chem. 2009 Nov;55(11):1900–4. [PubMed: 19797713]


Will Taking Testosterone Reduce Gynecomastia
Source: https://www.ncbi.nlm.nih.gov/books/NBK279105/


0 Komentar